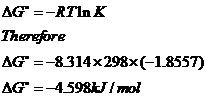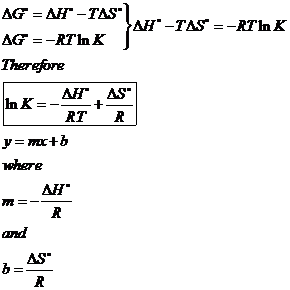Delta G delta G0 RTlnQ where Q productreactants expression. -390 2 -230 -50 2 0 Δ G.
When Equilibrium is obtained delta G 0 So delta G0 -RTln K ie.

What is delta g formula. If the specific heat of gold is 129 Jkgcdot k. CO 2g 2 H 2 O g CH 4g 2 O 2g Δ G. Entropy - the amount of disorder in the system.
Delta G Delta H - TDelta S Delta G 1105 kJ - 400 K1368 kjK Delta G 1105 - 5472 kJ 5578 kJ. Use the following formula. Chemical potential of component i in a mixture μ i.
What does it mean when Delta G is 0. If we know the standard state free energy change G o for a chemical process at some temperature T we can calculate the equilibrium constant for the process at that temperature using the relationship between G o and K. Energy of products Energy of reactants Δ G.
Delta Formula Example 3. In both cases R is the gas constant. CH 3 OHl -2386.
Enthalpy - the heat content of a system at constant pressure. Name of Species Delta HfkJmole Delta GfkJmole SJmole-K CO 2 g -3935 -3944 2137. G G o RT ln Q In this equation R is the ideal gas constant in units of Jmol-K T is the temperature in kelvin ln represents a logarithm to the base e and Q is the reaction quotient at that moment in time.
What is the general formula for Δ G. In order to avoid unit changes and keep Δ G in standard reasonable units R is usually given as 8314 J m o l K or even 0008314 k J m o l K. Q Δ G R T ln.
This form of the equation provides a useful link between these two essential thermodynamic properties and it can be used to derive equilibrium constants from standard free energy changes and vice versa. Modify the Δ H equation for this chemical equation. Delta G Delta H - T Delta S - Chad explains the relationship between Gibbs Free Energy Enthalpy and Entropy and when a reaction will be spontaneous.
Delta refers to the symbol of change Delta t refers to the change in temperature in kelvins K Solved Examples on Specific Heat Formula Example 1. Below is a table to summarize it up. When the Delta G for a reaction is zero a reaction.
Delta G is the symbol for spontaneity and there are two factors which can affect it enthalpy and entropy. Δ G Δ G R T ln. Δ H T Δ S Δ G is in the units Joules J.
Unfavorable reactions have Delta G values that are positive also called endergonic reactions. There are two main version of the Gibbs free energy equation that include R. Fill in the numbers for this specific equation.
When delta G 0 -. T is the temperature on the Kelvin scale. 8314 x 298 x ln281x10-16 -887x10 5.
G J ML 2 T 2. Rearrangement gives In this equation. In the article Gibbs free energy formula you will learn the meaning of Gibbs free energy standard Gibbs free energy change its unit derivation of Gibbs- Helmholtz equation ie Δ G Δ H T Δ S conditions of spontaneity the relation between free energy and equilibrium constant and many more.
Hence the Delta will be 00426. So delta G0 is not necessarily 0 be it at 25 deg C and 1 atmosphere or otherwise. Delta G 0 at equilibrium not delta G0.
Q is now K as Q was for non equilibrium. Then what quantity of heat energy is required to raise the temperature of. For a system at equilibrium Q K and ΔG 0 and the previous equation may be written as.
In chemistry delta G refers to the change in Gibbs Free Energy of a reaction. Gibbs Free Energy refers to the energy in a chemical reaction that can be used to do work. Given that delta G RTlnKd if you wanted to increase the dissociation constant by 100x at physiological temperature of 310K would you have to increase or decrease delta G by 27kcal mol.
Δ S is in the units of Joules per Kelvin J K. JP Morgan is one of the biggest investment banks Biggest Investment Banks Top 10 bulge bracket investment banks are - Blackstone Goldman Sach co Morgan Stanley JP Morgan Chase co Bank of America Merrill Lynch Credit Suisse Citi Deutsche Bank HSBC UBS. The deviation of delta G from delta G0 is given by.
If you want to calculate DeltaG under non-standard conditions you need to use the equation DeltaG DeltaG0 RTlnQ where Q is the ratio of concentrations. T is in the units of Kelvin K. Enthalpy Temperature Entropy Δ G.
The relationship between the free energy of reaction at any moment in time G and the standard-state free energy of reaction G o is described by the following equation. Where Delta n_i is the change in the amount of compound i in moles and G_mathrmmi is the molar Gibbs free energy of the pure compound i under the T and p conditions specified. In general one could write for a chemical reaction Delta G sum_i Delta n_i G_mathrmmi.
Read more in the United States of America. Thus the mass of the gas ejected is delta m If law of conservation of linear momentum is to hold the momentum of the system at time t momentum of the system at time t delta t Or Momentum of rocket at time t Momentum of rocket with reduced mass Momentum of the gases. R 8314 J mol-1 K-1 or 0008314 kJ mol-1 K-1.
The Gibbs Free Energy of a reaction delta G can be calculated through the equation delta G delta H - Tdelta S. Because this reaction has a positive Delta G it will be non-spontaneous as written. Δ H is in the units of Joules J.
Δ G -800 kJmol. Solve for Δ H.

15 2 17 2 Delta G Theta Rtlnk Gibbs And Equilibrium Constant Calculations Hl Ib Chemistry Youtube

Gibbs Free Energy And Spontaneity Article Khan Academy

Free Energy Delta G And Equilibrium Pt 8 Youtube